Research
Evaluation of Material Properties of MEMS Devices and Development of the Evaluation Methods
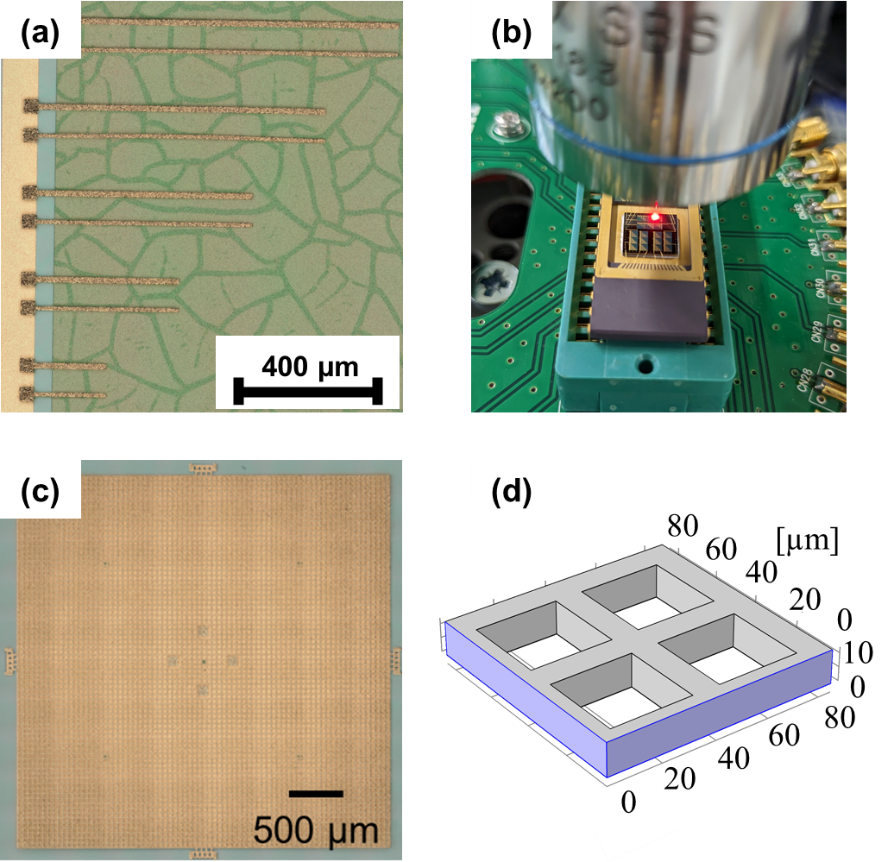
MEMS (Micro-electro-mechanical systems) devices, which integrate microscale sensors, actuators, and other mechanical components onto a single chip, are widely used in our daily lives. Sone-Chang’s group focuses on the development of MEMS accelerometers with high sensitivity composed made from electrodeposited-Au materials. To achieve this, we study the evaluation of the material properties and reliability of electroplated gold and the development of evaluation methods for these properties. For example, we evaluate the long-term structural stability and effective Young’s modulus of electrodeposited-Au micro-cantilevers, as shown in the figure below. We also conduct the evaluation of the structural stability of electrodeposited-Au components installed into MEMS accelerometers with high sensitivity by experiments and finite element analysis (FEA).
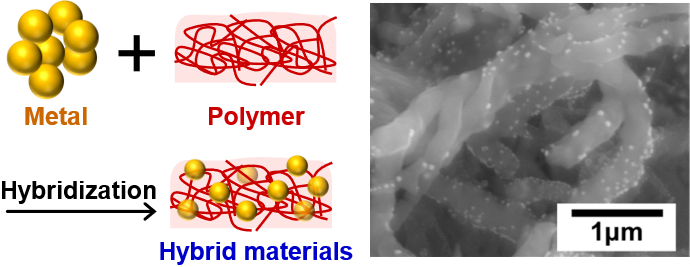
Development of Hybrid Materials and their Applications
Hybrid materials, composed of several materials with different properties, are expected to exhibit unique functions that cannot be achieved by single materials, due to the synergistic interactions between different materials. For example, hybrid materials composed of noble metals, such as gold or platinum, and conducting polymers, such as polyaniline or polypyrrole, are attractive as electrode materials for electrochemical glucose sensors. Their functions are tuned by changing various parameters, such as types and combinations of materials, sizes and shapes of materials, and their fabrication processes. This versatility allows researchers to reflect their originality and ideas. Sone-Chang’s group focuses on the development of metal/polymer composites and noble metal/metal oxide/conducting polymer materials using electrochemical approaches.
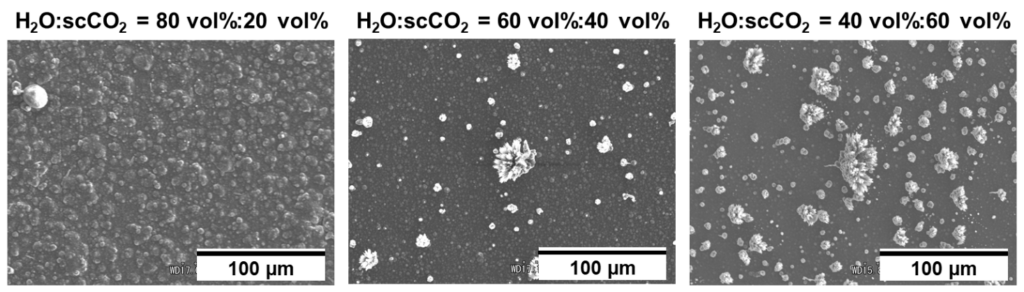
Development of Environmentally Friendly Electrosynthetic Platform Using Supercritical Carbon Dioxide
Electrosynthesis is an environmentally friendly chemical reaction process that carries out redox reactions without chemical oxidants or reductants. The choice of the solvent is important to perform electrosynthesis. Water is a low-cost and green solvent, indicating that electrosynthesis performed in water would be promising for a greener process. However, the applicable substrates remain limited, due to various problems, such as their solubility in water and its potential window. To overcome these problems, Sone-Chang’s group focuses on using supercritical carbon dioxide (scCO₂), which is also recognized as a green solvent, as a co-solvent, and aims to develop a novel environmentally friendly electrochemical platform based on the scCO2-emulsified electrolyte. Recently, we have demonstrated the electrodeposition of the conducting polymer polypyrrole using this system. Changing the volume ratio of water to scCO₂ resulted in polypyrrole with different morphologies and provided unique morphologies that were not observed previously. The reaction mechanisms and applications of this system are currently being studied.